

Hence, there are three types of covalent bonds:.When two atoms share three pairs of electrons, a triple covalent bond is formed.When two atoms share two pairs of electrons, a double covalent bond is formed.When two atoms share one pair of electrons, a single covalent bond is formed.By doing so, the two atoms share 1, 2 or 3 pairs of electrons so as to achieve stable noble gas electron arrangements.During the formation of a covalent bond between two atoms, each atom contributes 1, 2 or 3 electrons to each other for sharing.The sharing of electrons between the two atoms takes place in such a way that both the atoms acquire the stable electronic configurations of their nearest noble gases.The chemical bond formed when two atoms share electrons between them is known as a covalent bond.The shared pairs of electrons which bind the atoms together are called covalent bonds.Īs a result, covalent molecules are formed.Each atom contributes the same number of electrons to each other for sharing.During the formation of covalent bonds, atoms of non-metals share electrons to achieve stable noble gas electron arrangements.Hydrogen forms covalent bonds when it combines with more electronegative non-metal such as a fluorine, oxygen, nitrogen, chlorine, bromine, iodine or carbon. (b) carbon and silicon from Group 14 of the Periodic Table. (a) the elements from Groups 15, 16, and 17 of the Periodic Table.
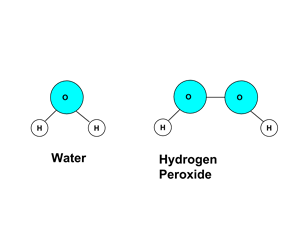
Non-metal + Non-metal → Covalent compound Covalent bonds are formed when atoms of non-metals combine with each other to form a molecule.


 0 kommentar(er)
0 kommentar(er)
